About EnLyte-D
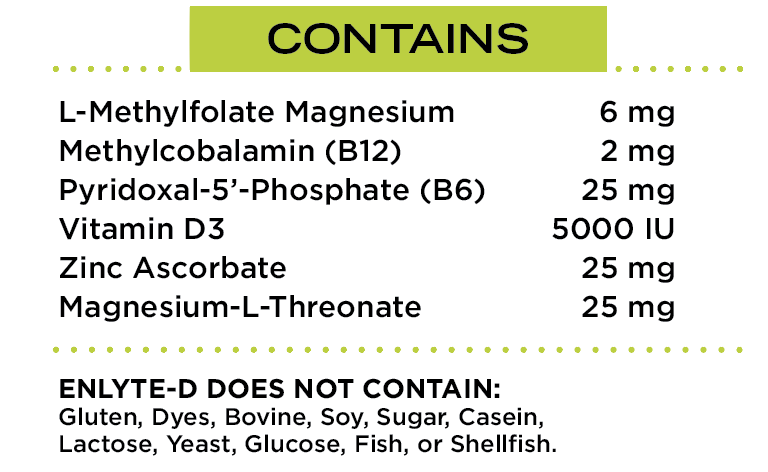
EnLyte-D is a FDA REGULATED MEDICAL FOOD to be used under medical supervision that has been specifically formulated for patients with methylenetetrahydrofolate reductase (MTHFR) genotypes and/or genotypes in Vitamin D transport and other B-Vitamin polymorphisms that may contribute to hyperhomocysteinemia and/or Vitamin D deficiency. EnLyte-D provides patients special, medically determined nutrient requirements including L-Methylfolate, Vitamin D, and other B vitamin coenzymes and mineral co-factors in amounts that cannot be achieved by the modification of the normal diet alone.
EnLyte-D is indicated for patients with distinct nutritional requirements for the dietary management of certain metabolic processes and IEMs (Inborn Errors of Metabolism) identified with hyperhomocysteinemia that contribute to: Major Depressive Disorder, Pain, Tingling & Sensation Related To Diabetic Peripheral Neuropathy, Dementia, Cognitive Dysfunction And Alzheimer’s Disease, Addiction And/Or Abuse Abatement, Early & End Stage Renal Disease, Migraine With Or Without Aura, Age-Related Macular Degeneration.

